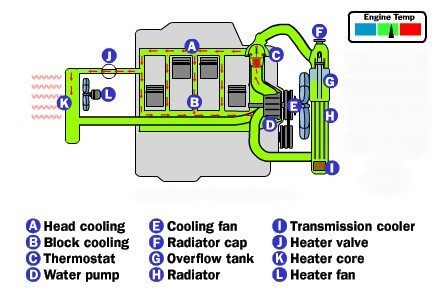
Coolant
Cars operate in a wide variety of temperatures, from well below freezing to well over 100 F (38 C). So whatever fluid is used to cool the engine has to have a very low freezing point, a high boiling point, and it has to have the capacity to hold a lot of heat.Water is one of the most effective fluids for holding heat, but water freezes at too high a temperature to be used in car engines. Water boils at 120 degrees so water alone will not work under extreme heat either. The fluid that most cars use is a mixture of water and ethylene glycol (C2H6O2), also known as antifreeze. By adding ethylene glycol to water, the boiling and freezing points are improved significantly. The normal mixture is a 50/50 blend.

The temperature of the coolant can sometimes reach 250 to 275 F (121 to 135 C). Even with ethylene glycol added, these temperatures would boil the coolant, so something additional must be done to raise its boiling point. Anti-freeze with out water will also boil before the engine reaches operating temperature so water must be mixed in.
The cooling system uses pressure to further raise the boiling point of the coolant. Just as the boiling temperature of water is higher in a pressure cooker, the boiling temperature of coolant is higher if you pressurize the system. Most cars have a pressure limit of 14 to 15 pounds per square inch (psi), which raises the boiling point another 45 F (25 C) so the coolant can withstand the high temperatures.
Antifreeze also contains additives to resist corrosion and provides lubricant for the water pump bearings.